NANOMIZER Inc.
| Nano-Emulsion Fuel Technology |
Nano-Emulsion Fuel Technology
Emulsion fuel is a blend of existing fuel oil (heavy oil, light oil, kerosene etc) mixed with about 10-30% water. It is a new type of fuel used in internal combustion chambers such as engines, boilers etc and can reduce fuel consumption and emissions like NOx, PM and smoke by improving fuel efficiency.
It was first used in the US more than 40 years ago and many different types have been tested in Japan as well. Although it was commercialized as a new type of fuel about 10 years ago in the US and Europe with government support such as tax incentives and subsidies, currently its use has not been fully established yet.
■ Development of Nano-Emulsion Fuel
Nanomizer Inc. has been investigating the problem of why emulsion fuel has not achieved widespread usage despite its long history and promise. We have been developing nano-emulsion fuel from about 5 years ago. As a result, we have overcome the problems of conventional emulsion fuel by making it possible to reduce fuel consumption in engines, industrial furnaces etc, thereby reducing cost, CO2, NOx and so on. Our nano-emulsion fuel is a next generation new fuel that is completely unlike conventional emulsion fuel.
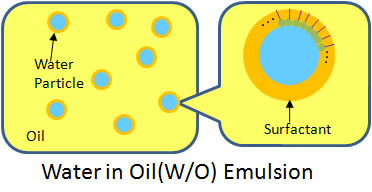
■ Conventional Emulsion Fuel
Emulsion fuel is a blend of water added in fuels such as heavy oil, light oil and kerosene. Generally, the water particles are dispersed within the oil. Depending on the type of fuel, suitable additives may be needed.
| Oil Category | Light Oil, A Heavy Oil, Kerosene, Recycled Oil |
Bunker Oil, C Heavy Oil, Coal Tar | Vegetable Oil (Next Generation Fuel) |
| Additives | Required | Not Required | Required |
| Users | Automobiles, Boilers, Medium and Small Power Generators, BR※, Small and Medium Vessels | Large Vessels, Medium and Large Power Generators, Large Boilers |
Expected to be used by Power Generators and Ship Engines |
| Viscosity | Low | High | High |
| Operating Temperature | Room Temp. | High Temp. (90~140 degree) |
Room Temp.~ High Temp. |
※BR : Burning Reactor means Burner used for Industrial Furnace
【 Emulsion Fuel Mechanisms 】
When emulsion fuel is injected into an engine or combustion chamber as a liquid, it is exposed to a high temperature. Water, which has a lower boiling point, will then vaporize and expand by 1700 times in volume, thereby dispersing the surrounding liquid oil. Due to the micronization of the oil, the contact surface area with oxygen (air) increases which improves the combustion efficiency and fuel consumption. As a result of the more complete combustion, PM and smoke are also reduced greatly which leads to a reduction in emission gases.
Mechanism |
Effects |
|
1:
|
Due to the difference in boiling points, water expands or explodes first (volume approx. 1700 times). Subsequently, the particles of oil are particulated, and forced blending with the air occurs, thereby enabling efficient and complete combustion.
|
▼reduction in fuel |
2:Cooling |
Ordinarily, NOx is generated when the air is exposed to high temperatures. Water vapor dilutes the reactive region and air is cooled, thereby reducing the NOx. |
▼reduction in NOx |
3: |
The overall mass increases by adding water which has a higher density, thereby increasing the momentum and improving the mixture with the air. |
▼reduction in fuel |
4: |
The fuel contacts the air, and the non-reacted carbon reacts with water, thereby supplementing imperfect combustion. |
▼reduction in fuel |
In addition, due to the latent heat capacity of water, the internal cylinder temperature of the engine is lowered. This suppresses the oxidation of nitrogen in the air and causes the generation of NOx to drop. In the combustion chamber, the carbon reacts with the oxygen in the water in what is known as a water-gas reaction which reduces substantially the amount of air required from the outside. Consequently, there is no cooling in the internal chamber which improves the heat efficiency.
In theory, emulsion fuel is therefore effective in reducing emission gases and fuel consumption. As a result, many researchers including some large companies have been developing emulsion fuels for the last 40 years.
■ Issues with Conventional Emulsion Fuel
There are 2 main issues with conventional emulsion fuel.
① Insufficient fuel savings
The fuel savings from using conventional emulsion fuel is not very high and is less than a few % at best. This can be attributed to the inadequate water vapour explosion and water-gas reaction. In particular, for bunker fuel emulsion used by large vessels in the shipping industry, the fuel efficiency has been reported to become worse.
② High cost of additiveExcept for certain fuels such as C-Heavy Oil, an additive (surfactant) is required to be added in order to maintain the stability of the emulsion. For conventional emulsion fuel, 3-10% of additive is required and this makes the emulsion fuel more expensive than the original fuel despite adding 10-30% water in the emulsion fuel. Therefore conventional emulsion fuel could only be used as an expensive way of reducing emission gases.
![]()
Conventional emulsion fuels have no fuel savings effect
and have been commercialized as merely environmental measures
against emission gases in Europe and the US with the help of government subsidies.
【 Our Nano-Emulsion Fuel Approach 】
■ Nano-Emulsion Fuel
【 Comparison between Emulsion Fuel and Nano-Emulsion Fuel 】
The biggest difference between our nano-emulsion fuel and other emulsion fuel is in the size of the water particles. Compared to a water particle diameter of about 10 μm in conventional emulsion fuel, the size of the water particles in our nano-emulsion fuel is only about 300 nm. As a result, even with the same amount of water added, our nano-emulsion fuel contains 37,000 times more water particles with 33 times more contact surface area compared to conventional emulsion fuel. Consequently, the water-oil boundaries increase drastically and the effects of emulsion fuel are increased substantially.
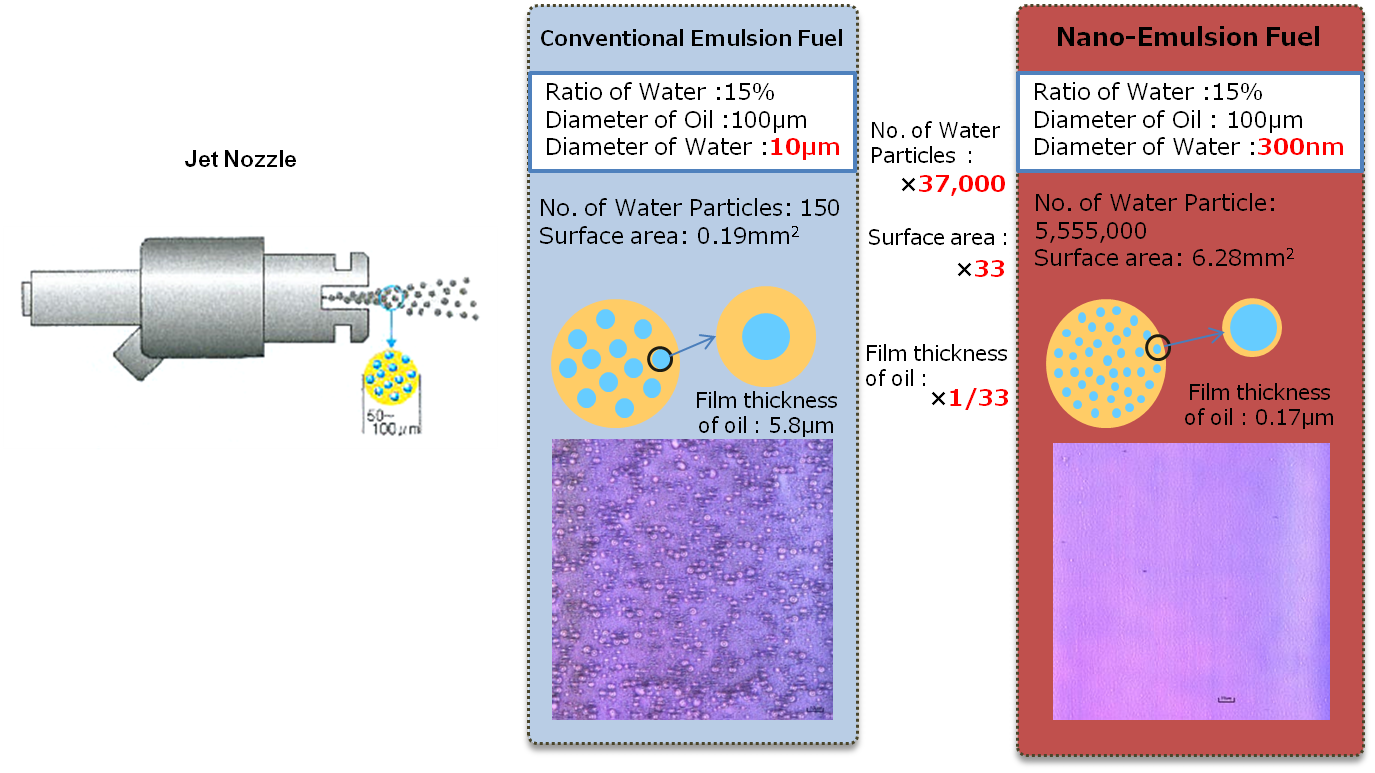
When the size of the water particles becomes smaller, the stability of the emulsion improves drastically. The separation of water and oil is largely due to the differences in relative weight (density) between the two. As a result, water which has a larger density, will precipitate, agglomerate and become one. In this case, the speed of the precipitation is directly proportional to the square of the diameter of the water particle according to Stokes Law. In other words, if the water particle size is 1/33 smaller, it means the speed of precipitation is 1000 times slower.
![]()
By pulverizing, dispersing and homogenizing the water particles
evenly in the emulsion fuel, the combustion efficiency is improved
and the fuel consumption reduced substantially.